Research
Chronic Stress Paradigms For Both Sexes
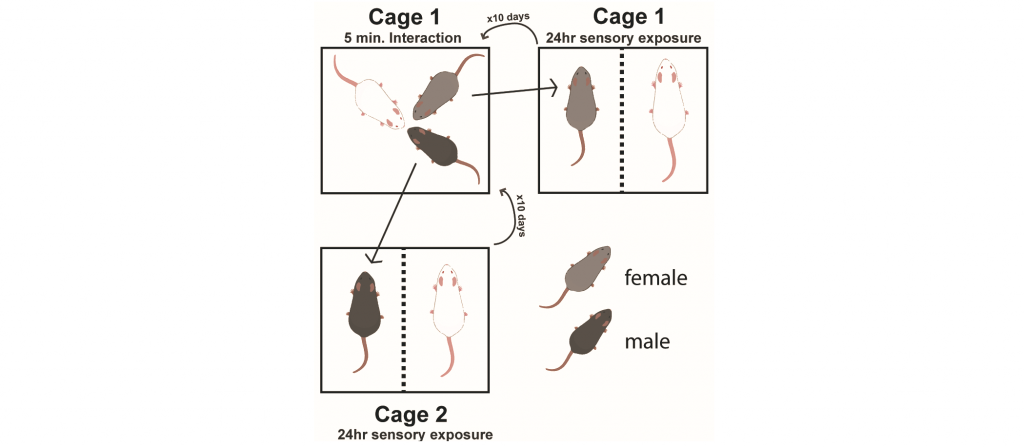
Chronic exposure to stress can precipitate mood disorders in humans. Therefore, chronic stress paradigms, such as unpredictable chronic mild stress, chronic social defeat stress, and chronic corticosterone administration are used to induce maladaptive affective states in rodents. However, these paradigms were designed in, optimized for, and only are effective in inbred strains of male rodents. However, women have a higher incidence of mood disorders than men. Therefore, one focus of the lab is to develop chronic stress paradigms that are effective in both male and female rodents. In the chronic nondiscriminatory social defeat stress paradigm, simultaneous exposure of one C57BL/6J male and one C57BL/6J female to the home cage of an aggressive CD1 mouse results in defeat of both intruders. In the social instability stress paradigm, both male and female mice are exposed to unstable hierarchical cage conditions. Both these paradigms induce maladaptive avoidance behaviors and allow comparisons of chronic stress effects in males and females.
How Does Chronic Stress Affect Behaviors Associated With Motivation And Reward?
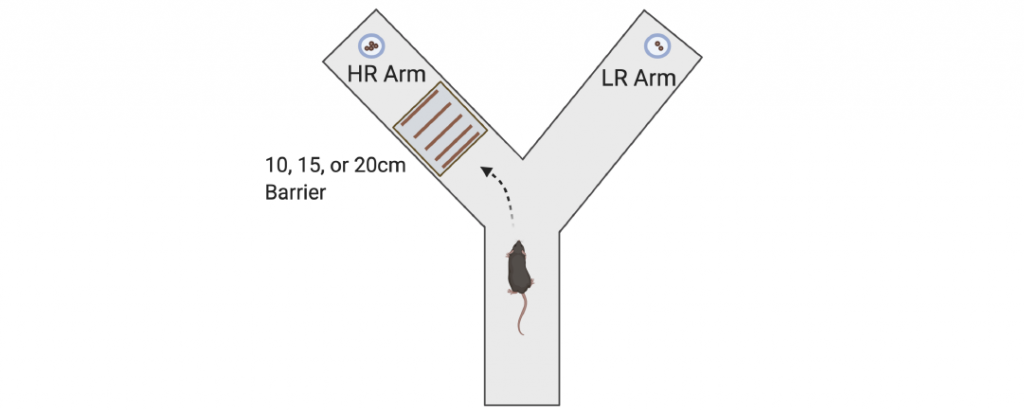
Another focus of the lab is to determine the effects of chronic stress on neural circuitry and behaviors associated with motivation and reward. Decades of work have shown that chronic stress affects limbic circuitry and results in maladaptive alterations in avoidance behaviors, such as open field, elevated plus maze, and novelty suppressed feeding. However, much less work has identified how chronic stress affects neural circuitry and behaviors associated with reward and motivation. In humans, mood disorders such as depression have symptoms related to anhedonia and low motivation. Importantly, very similar versions of reward and motivation behavioral tasks can be performed in both rodents and humans, with analogous data analyses and interpretations. These cross-species behavioral measures may provide enhanced translational validity and better guide therapeutic development than rodent avoidance behaviors historically associated with mood disorders. We recently reported that chronic corticosterone administration, which increases maladaptive avoidance behaviors, also impairs instrumental acquisition, reduces sensitivity to a devalued outcome, reduces breakpoint in progressive ratio, impairs performance in probabilistic reversal learning, and shifts effort-related choice behavior. We are currently expanding this area of research to incorporate the novel chronic stress paradigms we developed to determine whether there are sex differences in how chronic stress leads to deficits in reward processing and motivation. We are also using in vivo approaches to measure neural activity, such as fiber photometry (in collaboration with Dr. David Barker’s lab), in awake mice engaged in motivation and reward behaviors.
Neural Circuitry Underlying The Maladaptive Effects Of Chronic Stress
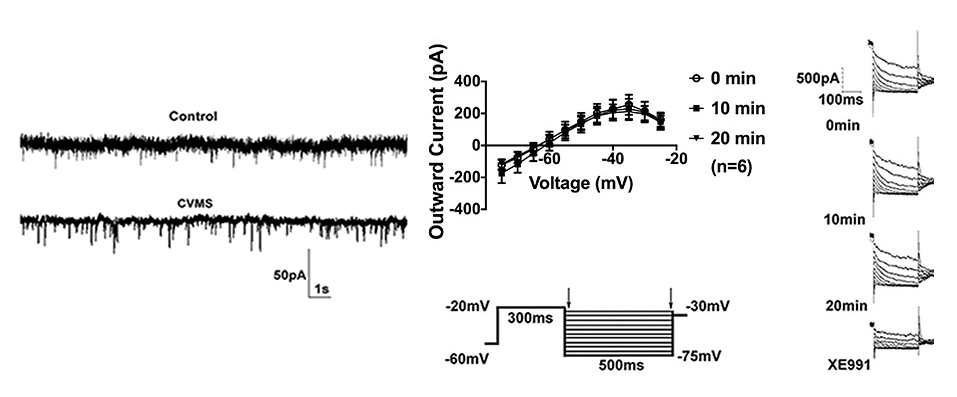
We are also working to understand the long-term effects of chronic stress on neural circuitry. One region that we are focusing on is the oval bed nucleus of stria terminalis. The bed nucleus of the stria terminalis (BNST) is a forebrain region highly responsive to stress that expresses corticotropin-releasing factor (CRF; also known as CRH) and is implicated in mood disorders. However, the exact mechanism by which chronic stress induces CRF-mediated dysfunction in BNST and maladaptive behaviors remains unclear. We recently reported that selective acute optogenetic activation of the oval nucleus (ovBNST) increases maladaptive avoidance behaviors in male mice. In collaboration with Dr. Troy Roepke’s lab, we also found that a 6-week chronic variable mild stress (CVMS) paradigm resulted in maladaptive behaviors and increased cellular excitability of ovBNST CRF neurons by potentiating mEPSC amplitude, altering the resting membrane potential, and diminishing M-currents (a voltage-gated K+ current that stabilizes membrane potential) in ex vivo slices. In another study we found that early life stress exposure results in long-lasting alterations in ovBNST that are similar to the effects of CVMS. In addition to mediating maladaptive avoidance behaviors, we hypothesize that ovBNST is also a key integratory site for stress and reward behaviors (although via distinct circuitry). Since BNST is sexually dimorphic, we are also interested in investigating effects of chronic stress on intra-BNST circuitry in both males and females.
Using Mice To Understand Antidepressant Treatment Resistance
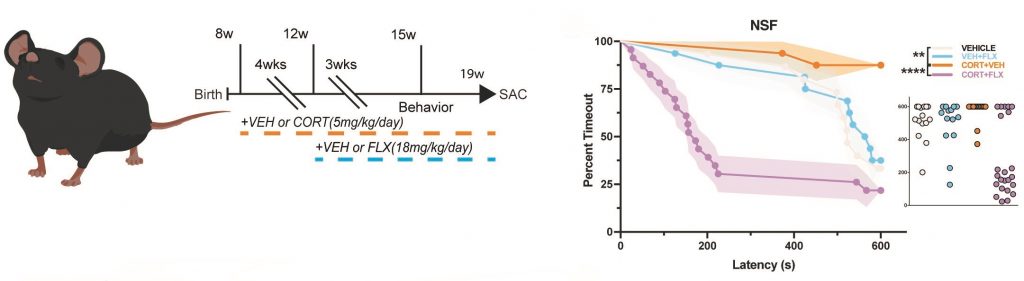
Another focus of the lab is to understand the molecular and neural circuit mechanisms underlying antidepressant treatment resistance. Chronic fluoxetine (FLX; an SSRI) treatment reverses chronic stress-induced behavioral changes in some, but not all mice, permitting stratification of mice into behavioral responders and non-responders to FLX. As a postdoc I published that 5-HT1A receptors, which are Gi-coupled inhibitory receptors, on mature granule cells (GCs) in the dentate gyrus (DG) are necessary and sufficient for the behavioral, neurogenic, and neuroendocrine response to chronic SSRI treatment. Since inhibition of mature DG GCs through cell autonomous Gi-coupled receptors is critical for mounting an antidepressant response, we assessed the relationship between behavioral response to FLX and DG GC activation in FLX responders, non-responders, and stress controls in both male and female mice. Using disparate stress paradigms, we found that male and female behavioral FLX responders show decreased DG GC activation (as measured by cFos immunostaining) relative to non-responders and stress controls. We then found in both sexes that chronic inhibition of ventral DG GCs (through usage of Gi-DREADDs) results in a decrease in maladaptive avoidance behaviors, while ventral DG GCs stimulation with Gq-DREADDs increases maladaptive behaviors. Finally, we were able to bidirectionally control the behavioral response to FLX through modulation of DG GCs. Chronic inhibition of ventral DG GCs with Gi-DREADDs converted FLX non-responders into responders, while activation of ventral DG GCs with Gq-DREADDs converted FLX responders into non-responders. We also performed an unbiased screen of gene expression in DG of responders and non-responders, where we found that Activin signaling in the DG is underrepresented in non-responders to SSRI treatment. Intriguingly, supplementation of Activin signaling in the dentate gyrus converts non-responders into responders and inhibition of Activin signaling converts responders into non-responders. Furthermore, Activin supplementation is a more effective augmentation therapy in non-responders than common second-line treatments.
Other Projects
Several other projects are ongoing, including assessing how chronic stress affects feeding behaviors and whether chronic stress exposure exacerbates phenotypes in adult mice with germline genetic deficiencies associated with neurodevelopmental disorders such as autism.